Is any special beverage likely to matter apart from water?
The biomineralization process resulting in a urinary stone has a multifactorial origin in which genetic, constitutional factors, metabolic abnormalities as well as diet and lifestyle might act in concert.
The supersaturation of urine with the stone-forming salt is a prerequisite for the necessary precipitation. A high fluid intake increases urine volume and reduces the risk of stone formation by lowering urinary activity product ratio (supersaturation) of stone-forming constituents.
Urine volume
A low urine volume is one of the most important risk factors for urinary stone formation. An adequate fluid intake is therefore an essential measure for the prevention of recurrent urinary stone formation, irrespective of stone composition. According to the Guidelines on Urolithiasis of the European Association of Urology (2015), the aim should be to obtain a 24h urine volume of at least 2.5 litres.
A randomized controlled trial in idiopathic calcium oxalate stone formers assigned participants either to increase fluid intake to maintain urine volume of greater than 2 l/d or to receive no treatment. During the five-year follow-up period, patients in the intervention group had significantly higher urine volumes, a 50% lower recurrence rate, and a longer time to first recurrence. Data from large observational studies repeatedly confirmed the inverse relationship between high fluid intake and the risk of stone formation in both men and women. A systematic review of 28 randomized controlled trials revealed that increased fluid intake substantially reduced the risk for recurrent calcium stones.
Urine pH and citrate excretion
Urinary pH is an important factor that triggers the formation of various types of stones in the urinary tract. An acidic urinary pH affects the solubility of uric acid and cystine, and promotes crystallization of the stone-forming components. The solubility of uric acid and cystine increases with a pH above 6.5 and 7.5, respectively. The dissolution of uric acid stones can be attained by urine alkalization at a pH of 7.0 to 7.2.
Hypocitraturia is an important risk factor for calcium oxalate urolithiasis. The amount of urinary citrate is mainly influenced by variations in the fraction of reabsorption. Changes in acid-base balance are the major determinants of urinary excretion of citrate. Ingested citrate is absorbed in the intestine and nearly completely metabolized to bicarbonate, providing an alkali load, which in turn increases urinary pH and citrate excretion. Thus, the effect of beverages on urinary pH and citrate excretion is mainly determined by the presence of bicarbonate and citrate, respectively.
Mineral water
Although mineral water has been suggested to be a suitable beverage for urine dilution, the water composition has to be taken into account. Bicarbonate (HCO3ˉ) is a natural component of mineral water.
Renal tubular H+ excretion is always coupled with the reabsorption of filtered HCO3ˉ. The ingestion of HCO3ˉ therefore increases buffering capacity of the organism and has a strong alkalizing effect.
The bicarbonate content of mineral water can replace alkalization therapy with potassium citrate and contribute to urine inhibitory power by increasing urinary pH and citrate excretion. A study in healthy subjects under standardized conditions revealed a significant and persistent increase in 24h urinary pH from 6.10 to 6.59 and citrate excretion from 3.045 to 4.554 mmol/24h after the intake of a mineral water containing 3,388 mg/l bicarbonate (Figure 1). During intake of the bicarbonate-rich mineral water, urinary pH values were significantly higher in each urine fraction compared to the control fractions (Figure 2).
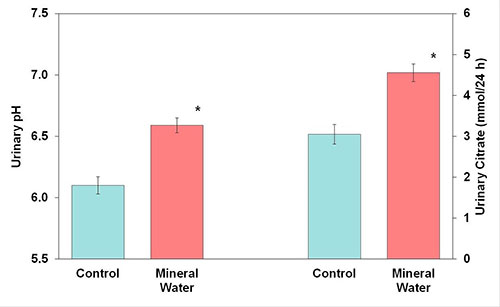
Figure 1: Urinary pH and citrate excretion before (control) and after receiving mineral water (M ± SEM; * P < 0.05)
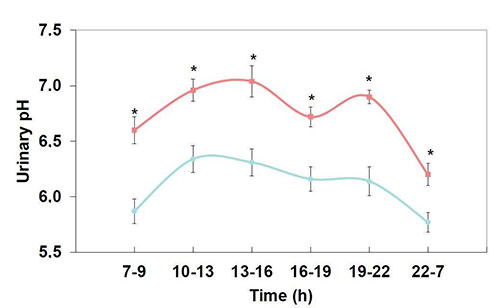
Figure 2: Diurnal variation in urinary pH during a 24 h period before and after receiving mineral water (* P < 0.05)
A randomized cross-over study in healthy individuals examined the effect of a bicarbonate-rich mineral water compared to a potassium citrate preparation on urine composition. The intake of 2 l/d of the mineral water containing 1,715 mg/l bicarbonate resulted in a significant increase in urinary pH from 6.06 to 6.68 and citrate excretion from 2.677 to 3.103 mmol/24h.
The effect of the bicarbonate-rich mineral water on urinary pH and citrate excretion was similar to that of potassium citrate, which was administered in equimolar concentration with respect to the alkali load (Figure 3). A double-blind cross-over study in 34 recurrent calcium oxalate stone formers compared the effect of a bicarbonate-rich mineral water with a water low in bicarbonate on urinary pH. During intake of 1.5 l/d of the bicarbonate-rich mineral water, a significant increase in urinary pH from 5.9 to 6.7 was observed.
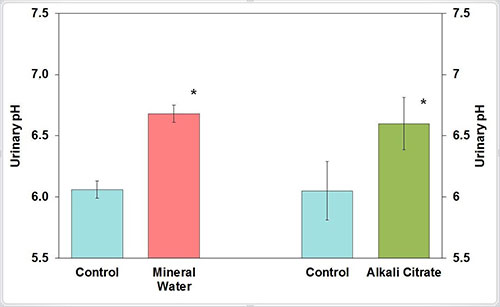
Figure 3: Urinary pH before (control) and after receiving mineral water or alkali citrate (M ± SEM; * P < 0.05)
Fruit juices
The effect of fruit juices is mainly determined by the presence of citrate. Citrus juices are rich sources of citric acid and potassium. Dietary citrate is absorbed in the intestine and nearly completely metabolized to bicarbonate. Bicarbonate can raise urinary pH and increase citrate excretion. Intracellular citrate is a central component of the Krebs cycle and provides the major part of the excreted citrate8. The amount of urinary citrate is determined by reabsorption.
Approximately 65 to 90% of the citrate filtered by the renal glomeruli is reabsorbed by the renal tubules. Changes in acid-base homeostasis appear to be the major physiologic determinant of proximale tubule reabsorption and urinary excretion of citrate. With alkali loads, urinary citrate excretion increases.
However, data from observational and interventional studies on the effect of different types of fruit juices on the risk of urinary stone formation are conflicting. The inconsistent results could be explained by differences in the populations and the dosing of potassium and citric acid. Analysis of the citric acid concentration of various fruit juices revealed a higher citric acid content of lemon and lime juice, both from the fresh fruit and from juice concentrates, than orange juice from the fresh fruit and orange juice, grapefruit juice and lemonade from ready-to-consume.
Soft drinks, coffee and tea
Since stone formers are advised to increase their intake of fluid, studies were undertaken to determine the effect of soft drink and coffee consumption on the risk of stone formation. However, data from clinical and epidemiological studies on the effect of different types of beverages on the risk of urinary stone formation are inconsistent. Whereas an interventional trial demonstrated an increased urinary calcium/ creatinine ratio and an elevated Tiselius risk index after caffeine loading, a prospective study found that coffee consumption was associated with a lower risk of stone formation.
Studies on the association between soda consumption and the risk of stone formation have likewise provided conflicting results. Assessing the data from two cohort trials, the intake of soda, including sugared cola, was not associated with increased risk for stone formation while results from three cohort studies revealed a higher risk of stone formation associated with consumption of sugar-sweetened cola.
Recommendations for stone formers
A sufficient circadian fluid intake by suitable beverages is one of the most effective nutritional measures irrespective of stone composition or the cause of stone formation. An adequate urine dilution is an important goal to reduce urinary supersaturation with lithogenic substances and to decrease the risk for stone formation. Depending on the environmental temperature and the degree of physical activity, it is usually necessary to drink at least 2.5 l/day to achieve the recommended 24h urine volume7. The fluid intake should be evenly distributed over the day.
It is particularly important to drink before going to bed at night to avoid increased urine concentration during the sleeping period. Patients with severe stone disease should be encouraged to have nocturia at least once per night. Patients exposed to chronic dehydration, caused by hot and/or dry environments, extensive physical activity or diarrhea, are recommended to replace extrarenal fluid losses.
The type of beverage should be carefully selected (Table 1). If the stone composition is unknown, neutral beverages should be preferred. Neutral beverages, that are fluids which dilute urine without affecting its composition, include tap water, mineral water with a low mineral content, fruit and herbal teas. Less suitable beverages are caffeinated coffee and black or green tea. Alcoholic beverages and sugar-sweetened soft drinks are unsuitable for stone formers. Fluids which additionally increase urinary pH and citrate excretion are bicarbonate-rich mineral water and citrus juices, for example orange and lemon juice.
Neutral and alkalizing beverages are suitable for the recurrence prevention of the majority of urinary stones that are calcium oxalate, uric acid and cystine. The calculation of the relative supersaturation of calcium oxalate suggests that bicarbonate-rich water can be an effective treatment option for the nutritional therapy of calcium oxalate stone disease (Figure 4). It should be emphasized that fruit juices have a considerable content of energy and should therefore be diluted with water before ingestion. Moreover, certain vegetable juices contain high oxalate concentrations.
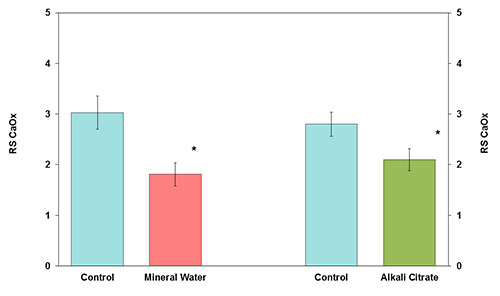
Figure 4: Relative supersaturation of calcium oxalate (RS CaOx) before (control) and after receiving mineral water or alkali citrate (M ± SEM; * P < 0.05)
Adequate fluid intake
A low urine volume is one of the most important risk factors for urinary stone formation. An adequate fluid intake, to achieve a urine volume of at least 2.5 l/24h, is the most important dietary measure for the prevention of recurrent urinary stone formation, irrespective of stone composition. Favourable changes in urine composition can be attained by special beverages. Beverage should be carefully selected.
Although mineral water has been suggested to be a suitable beverage for urine dilution, the water composition has to be taken into account. The bicarbonate content of mineral water can replace alkalization therapy with potassium citrate and contribute to urine inhibitory power by increasing urinary pH and citrate excretion.
The effect of fruit juices on urine composition is mainly determined by the presence of citrate. Citrus juices are rich sources of citrate. However, RCTs are lacking and data from interventional and cohort studies on the effect of different types of fruit juices on the risk of urinary stone formation are conflicting.
Caffeinated coffee, black and green teas are less suitable for urine dilution. Alcoholic beverages, including beer, and soft drinks, including cola, are unsuitable for recurrence prevention of urolithiasis.
Table 1: Recommendations for fluid intake
Neutral beverages
- mineral water with low content of bicarbonate, calcium and sulfate
- tap water (cave: pay attention to sterility of drinking water)
- herbal tea, fruit tea, kidney tea, bladder tea
- some fruit juices, g. apple juice
Alkalizing beverages
- mineral water with high bicarbonate content (at least 1,500 mg HCO3ˉ/l)
- citrus juices (e.g. orange, lemon, grapefruit juice)
Unsuitable beverages
- black tea, green tea
- caffeinated coffee (max. 500 ml/d)
- sugar-sweetened soft drinks, including cola
- alcoholic beverages, including beer
Author: Prof. Dr. Roswitha Siener
University Stone Centre Department of Urology University of Bonn, Bonn (DE)

