Using pharmacotherapy for OAB: What drug interactions to be aware of
By Prof. Martin C. Michel (Mainz, DE), Department of Pharmacology, Johannes Gutenberg University
This article reflects the highlights of the lecture Prof. Martin C. Michel will be giving during Plenary Session 02: “Optimal management of incontinence in the elderly patient” at EAU21 Virtual. This session takes place in Virtual Room 3 on Friday 9 July, 10.30 – 12.00 CEST.
The average patient seeking treatment for overactive bladder (OAB) is in his/her 60s. Most patients in this age group suffer from multiple diseases and, accordingly, receive multiple medications. This creates a potential for drug-drug interactions (DDIs) that may lead to altered efficacy and/or increased side effects. While urologists are trained to select medical treatment for OAB and other conditions for an optimal balance between efficacy and tolerability, the impact of comedications is often overlooked.
The prevalence of many diseases increases with age, for instance, OAB, benign prostatic hyperplasia (BPH), coronary heart disease, type 2 diabetes, or most types of cancer. Thus, older people are likely to suffer from multiple concomitant diseases. Most of them are treated at least partly by medication. Accordingly, the concomitant use of multiple drugs is frequent, and the prevalence of receiving multiple drugs concomitantly increases by age. For instance, based on the 2019 German claims data, only 6.5% of subjects aged 65 and older did not receive any prescription medicine. However, the median number of drugs used in this age group was seven (interquartile range 4-11), and 72.6% of patients received at least five different medications, i.e. exhibited polypharmacy. [4]
As patients receiving OAB medication by average are in their 60s, about half of all patients receiving OAB belong to the age group having by average seven different medications. This creates a major potential for DDIs. DDI can have a major impact on health: For instance, meta-analysis has shown that DDI account for 22.2% of all adverse event-related and 1.2% of total hospital admissions. [2]
DDI comes in two forms: pharmacodynamic and pharmacokinetic DDI. The former occurs if two or more drugs act on the same physiological system. Depending on what the drugs do, this can lead to greater or smaller effects. This can affect both efficacy and tolerability of the interacting drugs. Such interaction can be intentional when we use multiple drugs acting on the same organ (but mostly on a different molecular target) in many medical conditions to increase efficacy. An example of this is when treating urinary tract infections with a combination of antibiotics or bladder cancer with a treatment regimen based on multiple drugs. However, such combinations can also attenuate drug efficacy. For instance, acetylcholinesterase inhibitors are used in the symptomatic treatment of dementia. These inhibitors prevent the inactivation of acetylcholine which in turn, may enhance existing bladder overactivity and thereby limit the efficacy of OAB medications. Pharmacodynamic DDI typically affects all drugs using the same mechanism of action in a similar way.
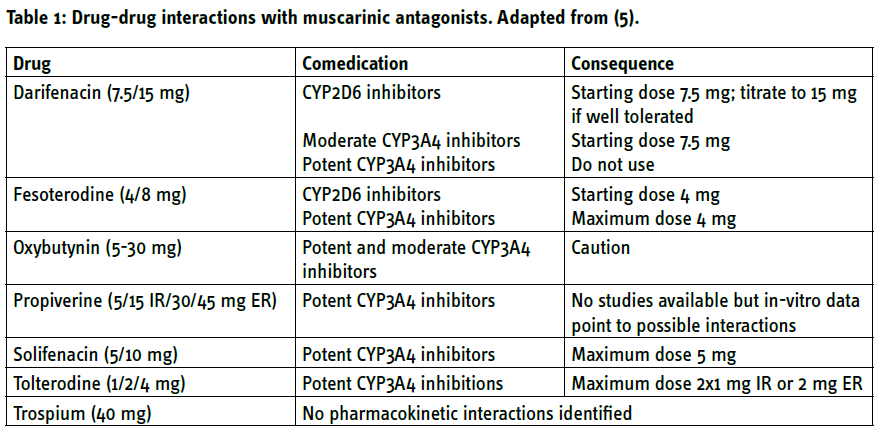
Pharmacokinetic DDI occurs when one medication affects the absorption, distribution, metabolism, or excretion of another drug. The most frequent form of this is one drug being metabolised by enzymes of the cytochrome P450 (CYP) system and another drug either inducing such enzymes or inhibiting them. This leads to reduced and enhanced exposure of the first drug, respectively. For instance, the anti-epileptic drug carbamazepine induces CYP3A4 which in turn, metabolises the immunosuppressant drug, cyclosporine. Cases have been reported where concomitant use of carbamazepine and cyclosporine led to underdosing of cyclosporine and loss of a kidney transplant. Pharmacokinetic DDI is not generalizable within a drug class, but specific for individual drugs (Table 1).
Muscarinic antagonists
Muscarinic receptor antagonists are the mainstay of medical OAB treatment. Side effects such as dry mouth and, to a lesser degree, constipation are frequent and unpleasant and often lead to premature discontinuation of treatment. Perhaps even more relevant are the cognitive side effects of muscarinic antagonists. [3]
While specific studies for such drugs in OAB are missing, it is generally accepted that the risk for side effects increases with the total number of medications having antimuscarinic effects. These include analgesics, antiarrhythmic drugs, antiemetics, antihistamines, antihypertensives, antiparkinsonian agents, antispasmodics, bronchodilators, ulcer drugs, many antidepressants and schizophrenia drugs. Generally, the higher the number of drugs with muscarinic antagonist properties, the greater the anticholinergic load and associated risk of cognitive impairment. [1] A comprehensive medication history is key to detect potential sources of pharmacodynamic DDI and prevent or reverse corresponding side effects.
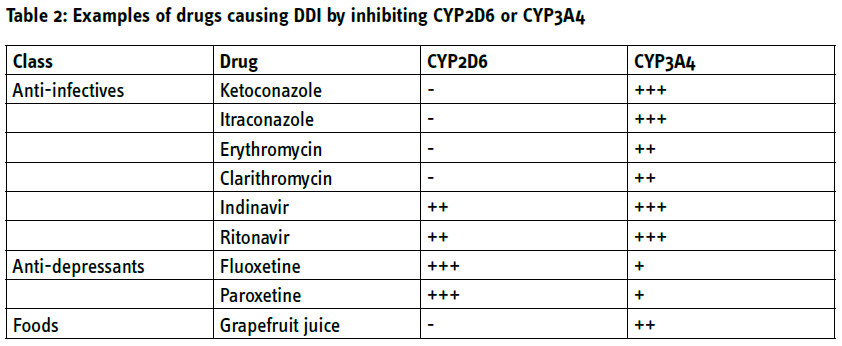
While there are no known pharmacokinetic DDI for trospium, all other muscarinic antagonists are metabolised by CYP2D6 and/or CYP3A4. Concomitant use of medications that inhibit these enzymes can increase exposure to the antimuscarinics. This leads to specific recommendations on adjusted dosing schemes in the presence of such comedication (Table 1). Frequently used inhibitors of CYP2D6 include anti-viral protease inhibitors such as indinavir and selective serotonin uptake inhibitors such as fluoxetine or paroxetine (Table 2). Potent inhibitors of CYP3A4 include azole-antimycotics (e.g. ketoconazole), macrolide antibiotics (e.g. erythromycin), protease inhibitors and some selective serotonin-uptake inhibitors but also grapefruit juice (Table 2). For instance, concomitant use of such drugs can increase the exposure to darifenacin 10-fold. [5]
β3-Adrenoceptor agonists
The only β3-adrenoceptor agonist currently approved in Europe is mirabegron. In contrast, to muscarinic antagonists, mirabegron has little risk for clinically relevant pharmacodynamic DDI. Concomitant use of potent CYP3A4 inhibitors can increase exposure to mirabegron, but the extent of this interaction is of limited clinical relevance in patients with normal renal function. However, in case of concomitant minor to moderate impairments of renal function (GFR 60-89 or 30-59 ml/min/1.73 m2, respectively), the mirabegron dose should be limited to 25 mg if potent CYP3A4 inhibitors are used.
Consequences
OAB patients are in an age group where multiple comorbidities and comedications are likely. Dedicated research on DDI has largely focused on the interaction between two drugs. While the specific evidence on what happens if three or more drugs are used concomitantly remains scarce, it is safe to assume that the risk for clinically relevant DDI increases with increasing numbers of concomitantly prescribed drugs. There are two pragmatic key defences against DDI: a comprehensive medication history and staying alert for any sudden change in efficacy or tolerability of drugs and their possible association with a change in comedications.
The greatest risk for clinically relevant DDI in the treatment of OAB comes from muscarinic antagonists. While the risk for pharmacodynamic DDI applies to the entire drug class, that for pharmacokinetic DDI depends on the specific compound in question.
When a pharmacokinetic DDI is suspected, it can be helpful to switch to an antimuscarinic that is metabolised/excreted by other pathways and less vulnerable to a specific interacting medication (Table 1). If this is not possible or if a pharmacodynamic DDI is suspected, switching to a β3-adrenoceptor agonist can be considered. If the result of the DDI is highly relevant and neither approach is feasible, it should be considered to stop one of the medications being involved. Such decisions should be based on an exchange with the colleague responsible for the prescription of the other drug. Drugs prescribed by other physicians should not be discontinued without consultation with the prescribing colleague.
Tips for clinical practice
The important pointers to keep in mind are the following:
- A complete medication history is key to the prevention, detection, and management of DDI.
- If the efficacy or tolerability of a drug changes, changes of medication regimen should be checked.
- Consultation with other physicians treating a patient is required to optimally address medical needs arising from DDI.
The reference list can be made available to interested readers upon request by sending an email to: communications@uroweb.org.

