The PIONEER Big Data platform: How does it apply to prostate cancer?
By Dr. Susan Evans Axelsson (Malmö, SE), Clinical Coordinator PIONEER
This article reflects the highlights of the lecture Dr. Susan Evans Axelsson will be giving during the EAU Specialty Session “PIONEER prostate cancer platform” at EAU21 Virtual. This session takes place in Virtual Room 5 on Monday 12 July, 14.30 – 15.30 CEST.
To transform prostate cancer (PCa) outcomes we must do things differently. We have not seen a pragmatic change in the treatment of PCa in the last 10 years, thus PIONEER was formed to address this problem and more. PIONEER is one of four Innovative Medicine Initiatives disease-specific Big Data for Better Outcomes (BD4BO) projects. Its focus is on BD4BO in prostate cancer while the other three focus on Alzheimer’s disease (ROADMAP), haematologic malignancies (HARMONY) and cardiovascular disease (BigData@Heart).
Though these are disease-specific projects, they are all focused on the same themes, namely:
- to design sets of standard outcomes and demonstrate value
- to increase access to high-quality outcomes data
- to use data to improve value of health care delivery
- to increase patient engagement through digital solutions
The projects share the same goal: to maximise the potential of Big Data to empower meaningful improvement in clinical practice, disease-related outcomes, and healthcare systems across Europe.
PIONEER is the ‘youngest’ of the four BD4BO programmes and thus has had the opportunity to build on the successes and learn from the failures of other projects. Because of this, PIONEER is building a stable and sustainable platform that will assemble, standardise, harmonise, and analyse high-quality big data from diverse populations of prostate cancer patients across all stages of the disease to provide evidence-based results for improving decision-making by key stakeholders and strengthen prostate cancer care and management.
Patients have been involved from the very beginning.
Prior to the start of PIONEER, the members of the EAU Prostate Cancer Guidelines panel and other prostate cancer key opinion leaders identified 44 questions as important knowledge gaps in the field of prostate cancer. Based on these questions, a prioritisation survey was conducted among key opinion leaders including healthcare professionals, pharmaceutical companies, and prostate cancer patients. In the first round, 73 healthcare professionals and 57 patients participated. For the second round, 12 questions were added, and the survey was translated from English into French, German, Italian and Spanish. 49 healthcare professionals and 169 patients replied in the second round, highlighting patients’ willingness to be actively involved in what they feel are crucial gaps in their healthcare and to become the ultimate beneficiaries of improvements. This survey resulted in 56 prioritised and re-ordered research questions covering all stages of prostate cancer, ranked according to the highest percentage for ‘critically important’.
Different research questions require different types of data. To ensure the PIONEER database holds information relevant to PIONEER’s aims, we ask each data holder to fill in a PIONEER Study Fact Sheet. With these fact sheets we are able to get a better understanding of the data contained in each dataset. The Study Fact Sheet questions what key data (if any) is available concerning the following topics: clinical, treatment, lab results, imaging, epidemiologic, economic, and genomic. As of May 2021, PIONEER has collected 44 fact sheets from potential data partners.
Data sharing models
Flexibility is key! In order to offer more flexibility to the data providers, the PIONEER consortium has chosen to utilise a mix of both the federated (remote data) and central (importable data) data sharing models and will ask the data provider to choose which model they prefer to use or are required to use by local law or other restrictions/regulations. Importantly, PIONEER will only accept anonymised data in the federated and central models and thus no original patient level data leaves the site. All data is anonymised and standardised to the OHDSI OMOPcommon data model behind the data providers firewall to facilitate analysis. The anonymised data is then moved to a central repository for research (in case of the central model), or the analytical code is sent to the data source where it is run locally in their own safe haven and from where only aggregated results are shared (in case of the federated model). Regardless of the chosen model, data contributors have the right to decide which studies they want to participate in and thus also have the right to opt out of studies.
PIONEER is contributing to the paradigm shift in the care and management of men with prostate cancer across Europe by collecting and collating high-quality datasets from European and non-European data providers. Data sources include hospitals, pharma, research institutes, biobanks/OMICS, and biotech companies. Ninety-five data sources have been identified as possible contributors to the platform. As of May 2021, 11 datasets are available in the PIONEER platform and 21 datasets are in the process of being converted to the OHDSI OMOPcommon data model or do not require conversion to be added to the platform.
Within PIONEER, we are developing a unique analytical toolkit for the analysis of a variety of data sources containing clinical studies, claims data, electronic health records, etc. to produce data descriptions, statistics, data visualisations and predictive models to answer the identified research questions. Bringing big data into an environment where it can easily be queried in a scientifically valid method to address these critical questions about how patient management impacts patient outcomes is essential for the future health care delivery model.
Study-a-thon
In March 2021, PIONEER, together with European Health Data & Evidence Network (EHDEN) and Observational Health Data Sciences and Informatics (OHDSI) joined forces to kickstart the process of answering PIONEER research questions by holding a five-day study-a-thon. The aim was to use big data and big data analytics to determine the real natural history of prostate cancer patients managed with watchful waiting. This event attracted 245 participants from 20 countries and combined results from at least 19 datasets (>two million prostate cancer patients from PIONEER and non-PIONEER data through the OHDSI community) to answer this question. This first study-a-thon demonstrated that we can create standardised operational definitions of clinically relevant concepts across datasets that will become the fundamental building blocks for future analyses (e.g. watchful waiting, active surveillance, disease progressions, etc.). This will ultimately speed up the analysis and facilitate the replication of analyses across multiple datasets leading to the generation of bodies of publishable and meaningful evidence that will support guideline development and revisions and changes in clinical practice.
As the PIONEER platform continues to grow with more high-quality big data from diverse populations of prostate cancer patients across different stages of the disease, its potential impact to change the clinical practice and fuel a new era in prostate cancer care and management is immeasurable.
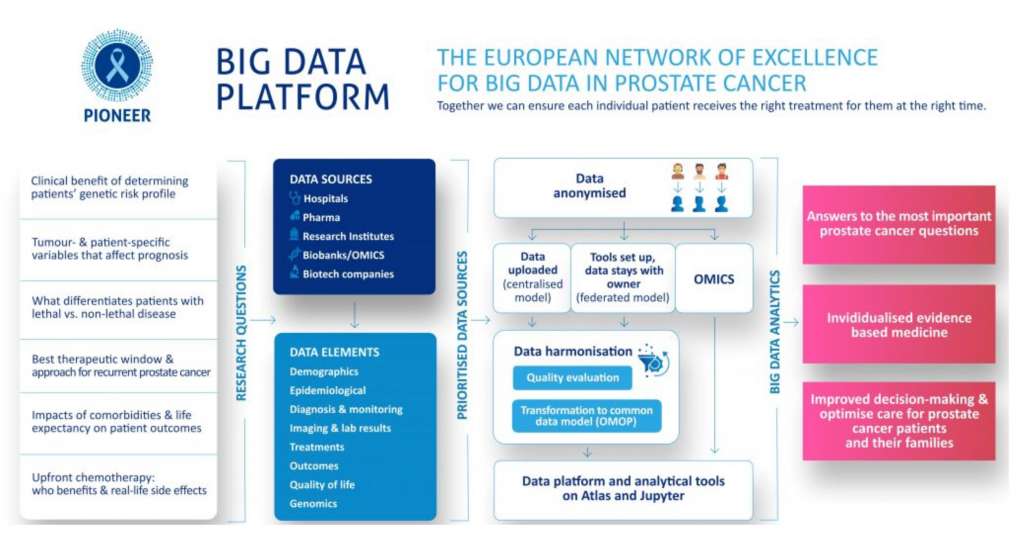
Figure 1: An infographic on the workings of PIONEER

